
- #Perchlorate ion bonding pairs how to#
- #Perchlorate ion bonding pairs full#
- #Perchlorate ion bonding pairs plus#
#Perchlorate ion bonding pairs how to#

Molecular geometry is the study of the physical shape of molecules. Silane, SiH4, has a terrible smell, but a delightful molecular geometry - tetrahedral! You can also find the three-dimensional shape in thiazyl trifluoride, NSF3, and ions of phosphate (PO43-), sulfate (SO42-), and perchlorate (ClO4-). But it is not the only molecule to make use of the familiar pyramid structure.
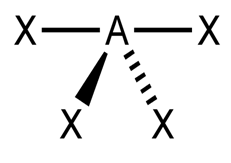
Methane is perhaps the most commonly found and familiar tetrahedral molecule. The result: a square planar molecule, not a tetrahedral. The four fluorine atoms take positions at four corners of a square. Xenon tetrafluoride, XeF4, has a steric number of six, not four it has two lone pairs that array themselves at 90° from the fluorine atoms (above and below the xenon atom) and 180° from each other. Molecular geometry, thanks to natural forces, seeks the lowest energy solutions to every bond, so some molecules with central atoms and four connected, surrounding atoms are not tetrahedral. The repulsion seeks its lowest energy level, providing the widest possible dispersal of the surrounding atoms. VSEPR theorizes that the lone pairs perform the same task as the bonds, repelling electrons to distribute joined atoms at equal angles around the central atom. For tetrahedral molecules like methane or xenon tetroxide, their steric number is four four bonds atom to atom and no lone electron pairs.
#Perchlorate ion bonding pairs plus#
All the bonds to the central atom, plus all the lone pairs, equals the molecule's steric number. Valence Shell Electron Pair Repulsion Theory, or VSEPR (pronounced "Vesper") predicts the molecular geometry of individual molecules. Lone pairs are the valence electrons of the atom that are not shared with another atom. The many shapes of molecules are affected by the number of atomic bonds and lone electron pairs. Valence Shell Electron Pair Repulsion Theory (VSEPR) It pushes the molecule into a three-dimensional structure.Ĭhemists have worked hard to explain the actual structure of molecules, developing a theory connecting geometry, energy, and atoms. Many shapes exist beyond tetrahedrals, but we are concentrating on that shape here.
We see the structure of molecules in chemistry connecting to geometry in the field of molecular geometry. Down to the scale of molecules, geometry still holds.
#Perchlorate ion bonding pairs full#
Geometry and real life are full of surprising alignments.


 0 kommentar(er)
0 kommentar(er)
